Clinical Trials
CLINICAL TRIALS
Introduction
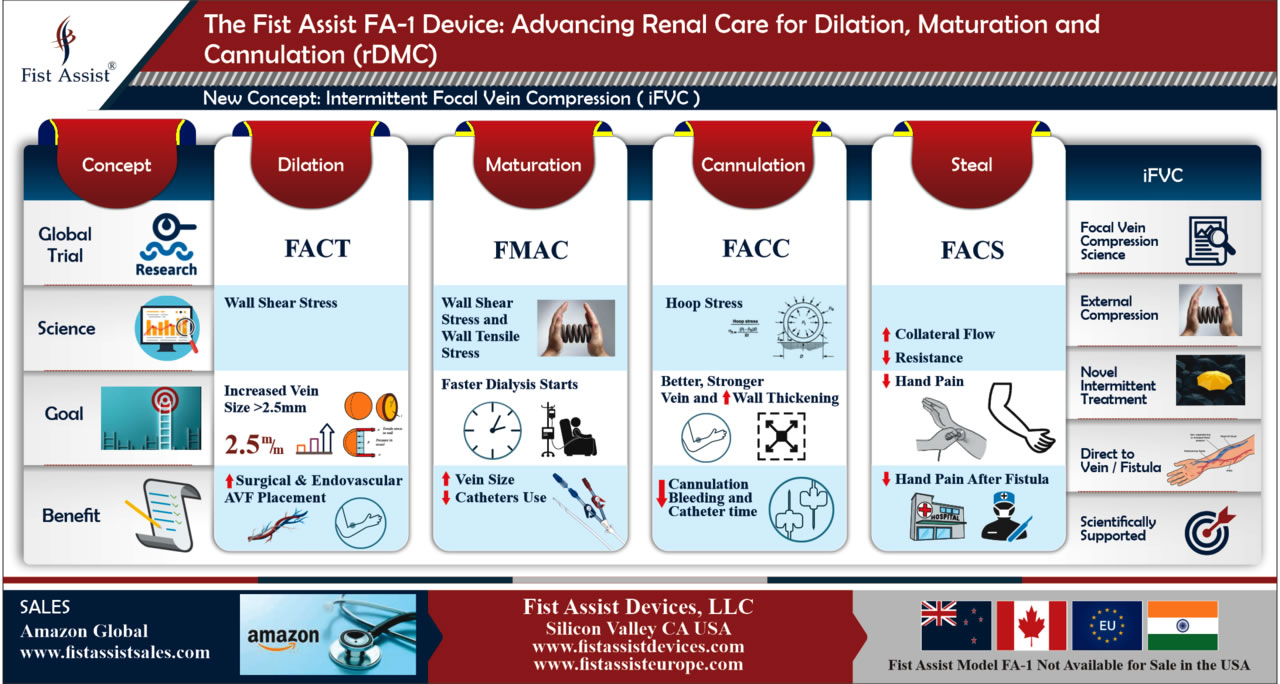
Delays in AV fistula (AVF) maturation can cause care delays and increased costs. Increased distention pressure and intermittent wall shear stress may dilate veins based on prior research. Early use of non-invasive devices may help assist clinical AVF dilation.
With the help of using psychological principle of urgency and lead generation technology - it resulted in one of the Beats Lead Generation Tool on the market!
With the help of using psychological principle of urgency and lead generation technology - it resulted in one of the Beats Lead Generation Tool on the market!
Methods
This was an IRB approved study. After AVF creation, a novel, intermittent pneumatic compression device `{`Fist Assist® (FA)`}` was applied 15 cm proximal to AVF to apply cyclic compression of 60 mm Hg for six hours daily for 30 days. Among the patients who completed one month follow up, thirty (n=30) AVF patients were in the study arm to test vein dilation with FA. Controls (n=16) used a sham device. Vein size was measured and recorded at baseline and after 30 days by duplex measurement. Clinical results (percentage increase) were recorded and tested for significance.
Results
No patients experienced thrombosis or adverse effects. Patient compliance and satisfaction was high. After one month, the mean percentage increase in vein diameter in the FA treatment group was significantly larger (p=0.05) than controls in the first 5 mm segment of the fistula after the anastomosis. All fistulas treated with FA are still functional with no reported thrombosis or extravasations.
Conclusions
Early application of an intermittent pneumatic compression device may assist in AVF dilation and are safe. Non-invasive devices like Fist Assist® may have clinical utility to help fistulae development and decrease costs as they may eventually assist maturation. Further studies directed to maturation are required.